




| 09:00-14:30 | Registration |
| 14:30-14:40 | Conference opening, T. Frederiksen |
| 14:40-16:20 | Mo1: Surface scattering and chemistry |
| 16:20-17:00 | Coffee break |
| 17:00-18:40 | Mo2: Solid-liquid interfaces |
| 19:00-21:00 | Welcome reception, Sala de Musica, Palacio Miramar |
Chair: W. E. Ernst, Graz, Austria
Invited talk
Liquid flow along a solid surface reversibly alters interfacial chemistry
1Max Planck Institute for Polymer Research, Ackermannweg 10, 55128 Mainz, Germany
2Physics Department, University of Namur, Rue de Bruxelles 61, 5000 Namur, Belgium
Water in contact with a solid surface is often in motion - e.g. water in riverbeds, rain drops falling on the ground, and pouring water in a glass. The influence of this motion on the structure of water at the interface is unclear. By using sum-frequency generation spectroscopy with an infrared and visible laser beam we can obtain the vibrational spectrum of the interfacial water molecules. We show by combining this technique with microfluidics that the interfacial structure is very sensitive to the motion of the liquid. Flow results in a reversible change of the surface charge and thus an alignment of the water molecules present at the interface. We observe this effect for calcium fluoride and silicondioxide interfaces at various pH. To obtain the same effect under static conditions, the pH of the bulk solutions has to be changed by up to 2 pH units. Moreover, we show that flow can invert the orientation of water molecules at the interface [1].
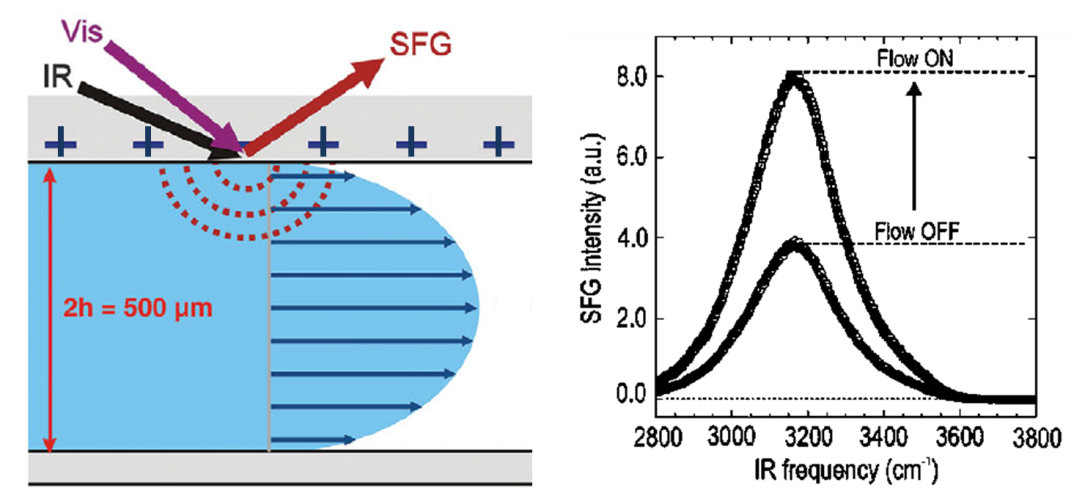
Figure 1: experimental geometry (left) and SFG spectra with the flow on and off for water at pH=3 underneath a CaF2 window (right).
[1] D. Lis et al., Science 344, 1138 (2014)