




| 09:00-14:30 | Registration |
| 14:30-14:40 | Conference opening, T. Frederiksen |
| 14:40-16:20 | Mo1: Surface scattering and chemistry |
| 16:20-17:00 | Coffee break |
| 17:00-18:40 | Mo2: Solid-liquid interfaces |
| 19:00-21:00 | Welcome reception, Sala de Musica, Palacio Miramar |
Chair: W. E. Ernst, Graz, Austria
Contributed talk
Spontaneous ferroelectric ordering of strongly correlated protons in crystalline ice films on Pt(111)
Department of Chemistry, Graduate School of Science, Kyoto University, Japan
Protons in ice are strongly correlated due to the ice rules [1]. The kinetic barrier of the synchronized proton motion at low temperature is so high that transition of bulk ice from the ordinary paraelectric state (proton disordered ice-I) to the ferroelectric state (proton ordered ice-XI) is practically prohibited without catalysts that promote proton transfer [2].
On the basis of the vibrational sum frequency generation (VSFG) spectroscopy [3], we report here substantial and extremely high-Tc ordering of strongly correlated protons in crystalline-ice films deposition on Pt(111). Heterodyne-detected VSFG (HD-VSFG) spectra revealed significant orientation preference of first-layer water molecules pointing toward substrate (H-down configuration) and the subsequent progressive propagation of the net-H-down orientation during multilayer deposition process (Fig.1a). The polarized ice film also showed a reversible and second-order like gradual thermal depolarization/repolarization during heating/recooling process with unexpectedly high critical temperature of Tc~180 K (Fig. 1b), proton dynamics of which is completely different from that in proton-ordered bulk ice-XI; irreversible and 1st-order depolarization to proton-disordered ice-I at Tc~72 K [2]. The emergent ordering is dominated by short range constraint of ice rules and their partial breaking due to the screening of polarization charges through the electron transfer at ice/Pt(111) heterointerface followed by the rearrangement of mobile protons.
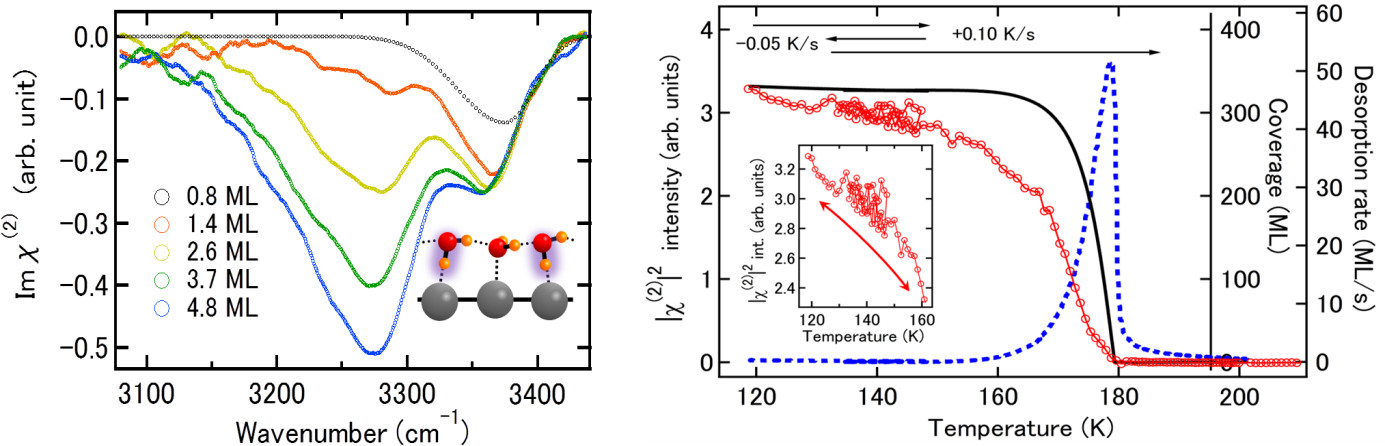
Figure 1. (a) HD-VSFG (Imχ(2)) spectra of OH-stretch band for HDO crystalline ice film deposited on Pt(111) at 140 K. (b) Simultaneous measurements of VSFG spectra and temperature programmed desorption (TPD). Red circle with red solid line, blue dotted line, and black solid line are VSFG, desorption rate (QMS-mass 19), and coverage, respectively, at each temperature for 330 ML crystalline-HDO-ice film.
[1] L. Pauling, J. Am. Chem. Soc. 57, 2680-2684 (1935)
[2] Y. Tajima, T. Matsuo, and H. Suga, Nature 299, 810 (1982)
[3] Y. R. Shen, Annu. Rev. Phys. Chem. 64, 129 (2013)