




| 09:00-14:30 | Registration |
| 14:30-14:40 | Conference opening, T. Frederiksen |
| 14:40-16:20 | Mo1: Surface scattering and chemistry |
| 16:20-17:00 | Coffee break |
| 17:00-18:40 | Mo2: Solid-liquid interfaces |
| 19:00-21:00 | Welcome reception, Sala de Musica, Palacio Miramar |
Chair: W. E. Ernst, Graz, Austria
Contributed talk
The motion of water on a hydrophobic surface
1The Cavendish Laboratory, JJ Thomson Ave., Cambridge, CB3 0HE, UK
2Institut Laue-Langevin, Grenoble, FR
3Department of Chemistry, University of Cambridge, Lensfield Rd., Cambridge, CB2 1EW, UK
4Department of Chemistry, University of Reading, Whiteknights, RG6 6AD, UK
We have prepared a system where we can follow the diffusion of water molecules on a picosecond timescale. While the desire to understand the behaviour of water at the nanoscale led to numerous experimental and theoretical studies [1,2], little is known when it comes to the dynamics of water from an experimental point of view [3,4].
By using helium-3 spin-echo spectroscopy [5] we are able to determine the motion of water on the system graphene/Ni(111). The hydrophobic nature of the substrate allows to measure the diffusion of water within a small temperature window (115 - 130 K) by applying an overpressure of water and hence maintaining a dynamic adsorption/desorption equilibrium.
The molecular dynamics extracted from spin-echo measurements shows thermally activated diffusion with a jump mechanism and an activation energy of 60 meV (see figure 2). The dependence upon the momentum transfer clearly shows jumps on a lattice where the preferred adsorption site of H2O is in the middle of the carbon hexagons (see figure 1). While this is in agreement with density functional theory (DFT) calculations, the energy landscape obtained from DFT is rather flat and cannot explain the experimentally determined activation energy. Furthermore, a characteristic rise at small momentum transfers indicates that interactions between the individual water molecules play a mayor role in the diffusion of water on graphene.
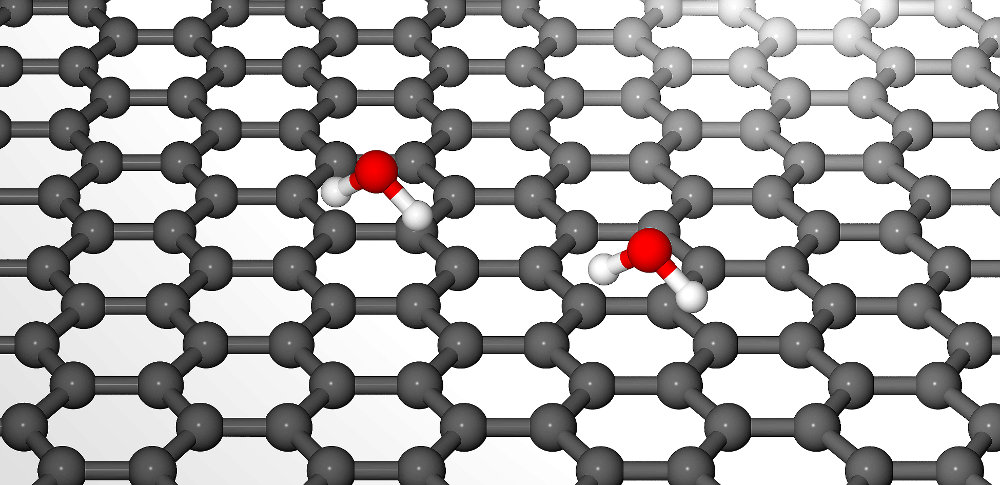
Figure 1: Graphical representation of two water molecules adsorbed on graphene. The preferred adsorption site is in the middle of the hexagon formed by the carbon atoms.
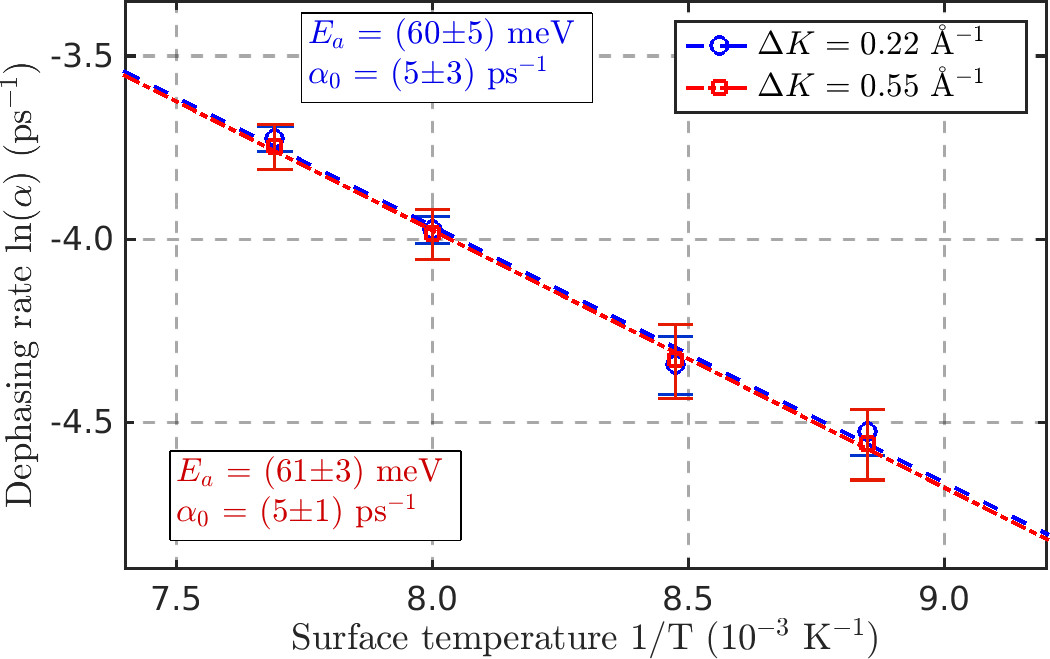
Figure 2: Arrhenius plot for the diffusion of water molecules on graphene/Ni(111) showing an activated process with an activation energy of 60 meV.
[1] A. Hodgson et al., Surf. Sci. Rep. 64, 381 (2009)
[2] J. Carrasco et al., Nat. Mater. Sci. 11, 667 (2012)
[3] K. Xu, et al., Science 329, 1188 (2010)
[4] J. Ma et al., Phys. Rev. B 84, 033402 (2011)
[5] A. Jardine et al., Prog. Surf. Sci. 84, 323 (2009)