




| 09:00-10:40 | Th1: Adsorbate and interface dynamics |
| 10:40-11:20 | Coffee break |
| 11:20-13:00 | Th2: STM-IETS and beyond |
| 13:00-15:30 | Lunch break (on your own) |
| 15:30-16:40 | Th3: Molecular films and 2D materials |
| 16:40-17:20 | Coffee break |
| 17:20-19:00 | Th4: Tip-enhanced vibrational spectroscopies |
| 20:30-23:00 | Conference dinner at Cofradía Vasca de Gastronomía, Old Town |
Chair: D. Farías, Madrid, Spain
Contributed talk
Protein dynamics and function from a physicist point of view
1Laboratoire ICB, UMR 6303 CNRS-UB, Université de Bourgogne, France
2Ecole Polytechnique Fédérale de Lausanne, Suisse
The directions of the largest thermal fluctuations of the structure of a protein in its native state are the directions of its low-frequency modes (below 1 THZ), named acoustical modes by analogy with the acoustical phonons of a material. The acoustical modes of a protein assist its conformational changes and are related to its biological functions. Low-frequency modes are difficult to detect experimentally. A survey of experimental data of low-frequency modes of proteins is presented. Theoretical approaches, based on normal mode analysis, are of first interest to understand the role of the low-frequency modes in proteins [1,2]. In this talk, we present applications of these methods to proteins intimately related to human diseases: ubiquitin and the 70kDa Heat-Shock Protein (Hsp70). The ubiquitin protein is a single domain protein which is a benchmark for biomolecular Nuclear Magnetic Resonance spectroscopy. Present all-atom calculations predict a "boson peak" near 20 cm-1 in the inelastic neutron scattering spectra of this protein. The molecular chaperone Hsp70 is an exemplary model to illustrate the different properties of the low-frequency modes of a multi-domain protein, which occurs in two well distinct structural states (open and closed states). The role of the low-frequency modes in the transition between the two states of Hsp70 is analyzed in detail. It is shown that the low-frequency modes provide an easy means of communication between protein domains separated by a large distance. [1]. Proteins can also subtend nonlinear excitations as we proven for the first time very recently by using molecular dynamics simulations and models from statistical physics [3]. How localized nonlinear excitations are related to the protein structure and sequence will be briefly described [3].
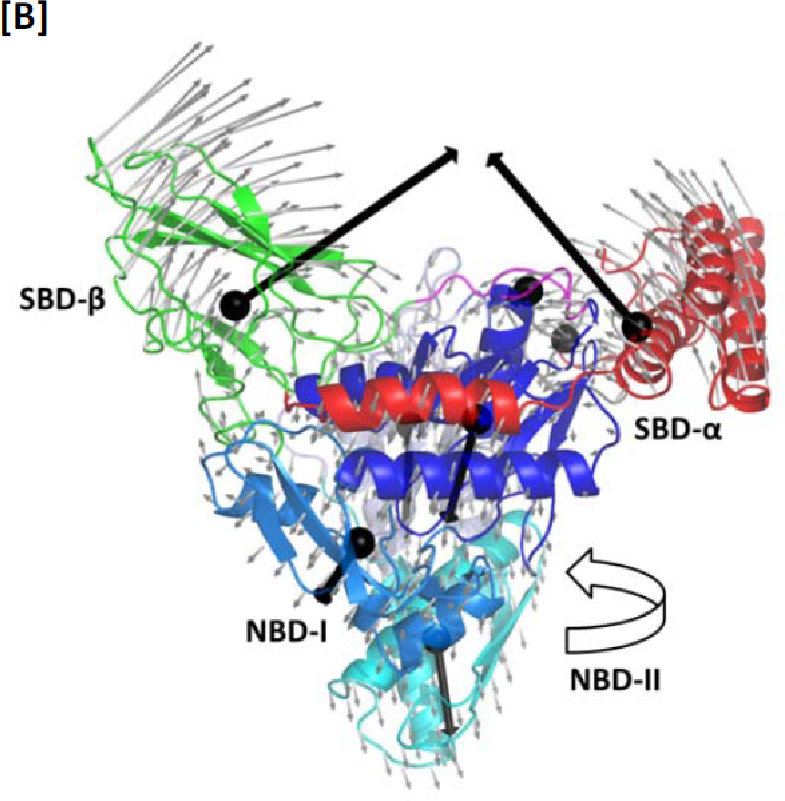
Figure 1: Typical low-frequency mode of the Human HSP70 Chaperone.
[1] A. Nicolaï, P. Delarue, P. Senet Chapter: Low-frequency, functional, modes of proteins: all-atom and coarse-grained normal mode analysis, in Comput. Methods on Study the Struct. & Dyn. of Biomolecules, Publisher: Springer-Verlag, Editors: A. Liwo, pp.483-524 (2014)
[2] F. Barakat, P. Delarue, P. Senet, unpublished
[3] A. Nicolaï, P. Delarue, P. Senet, submitted (2015)