




| 09:30-10:40 | Fr1: Thermal transport, friction, and dissipation |
| 10:40-11:20 | Coffee break |
| 11:20-13:00 | Fr2: Catalysis and single-molecule chemistry |
| 13:00-14:00 | Closing session with poster prize ceremony, G. Benedek |
Chair: M. Kawai, Tokyo, Japan
Contributed talk
Cold or hot: Metal substrate electrons will always couple to adsorbate vibrations
1Institut für Chemie, Universität Potsdam, Potsdam, Germany
2Donostia International Physics Center (DIPC), San Sebastián, Spain
3Leiden Institute of Chemistry, Leiden, The Netherlands
In recent years, a paradigm change began to take shape in experimental and theoretical surface science when it was realized that non-adiabatic effects, i.e., the coupling of electrons of a metal surface, say, to adsorbate degrees of freedom may have a decisive influence on their nuclear dynamics. In this contribution we present first-principles' based dynamical simulations and theory, demonstrating that indeed 'cold' and sometimes even more so, 'hot' electrons play a pronounced role during dynamical gas-surface encounters. In fact, non-adiabaticity seems the rule rather than the exception, certainly in the cases shown here.
In a first example, using the recently developed Ab Initio Molecular Dynamics with Electronic Friction (AIMDEF) method we show that vibrational relaxation of H atoms on metal (Pb) films is entirely dominated by (cold) electron-hole pairs. Phonon motion can nevertheless have some surprising impact, and so do Quantum Size Effects (QSE) which emerge with varying layer thickness [1]. Classical or open-system density matrix dynamics and generalized electronic friction models reveal the importance of 'cold' electrons during reactive and non-reactive molecule-metal surface scattering, as will be demonstrated for H2 near Ru(0001) and NO near Ag(111) [2]. Finally, 'hot' electrons created by femtosecond laser pulses play the music during photoreactions of adsorbates (diffusion, (associative) desorption), for systems like H2:Ru(0001) and CO:Ru (0001) [3].
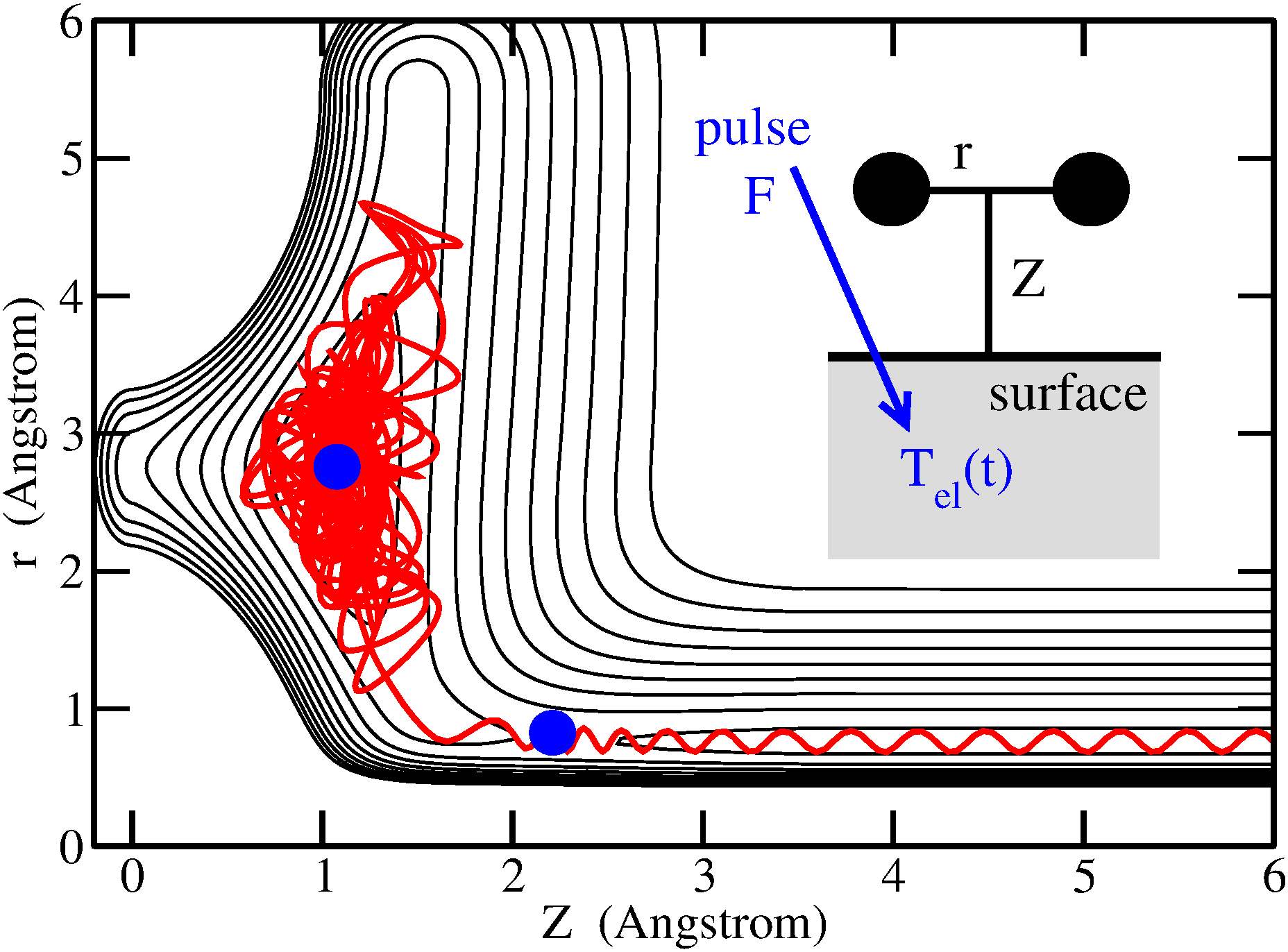
Figure 1: Example Langevin trajectory for hot-electron induced desorption of H2 from Ru(0001) [3].
[1] P. Saalfrank et al., J. Chem. Phys. 141, 234702 (2015)
[2] G. Füchsel et al., J. Phys. Chem. A 117, 8761 (2013); PRL 109, 098303 (2012); S. Monturet and P. Saalfrank, Phys. Rev. B 82, 075404 (2010)
[3] G. Füchsel et al., PCCP 12, 14082 (2010); PCCP 13, 8659 (2011); G. Floß et al., to be published