




Invited talk
From UHV to the solid/liquid interface: Water adsorption, surface reconstruction and dynamics on α-Al2O3
Fritz Haber Institute of the Max Planck Society, Berlin, Germany
α-Al2O3 surfaces exposed to water are ubiquitous in applications and a useful model for alumino-silicate surface/water interactions omnipresent in the environment. Despite this ubiquity, gaining a molecular level understanding of water interaction even with the crystallographically simplest α-Al2O3(0001) surface has proven challenging. Here we begin with the well defined α-Al2O3(0001) in ultra high vacuum and add controlled amounts water culminating in the solid/liquid interface. We characterize this system principally using time-averaged and ultrafast time-resolved vibrational sum frequency (VSF) spectroscopy in combination with a variety of electronic structure calculations. VSF spectroscopy is all-optical and interface specific by its symmetry selection rules and thus allows us to probe the spectral response of interfacial water, through its OH stretch, and surface Al-O vibrations (i.e. surface phonons) over 1010 range in water chemical potentials. This combination of sample preparation and characterization has allowed a series of novel insights: (a) we have experimentally probed the (heretofore only theoretically predicted) unimolecular water dissociative adsorption pathways and shown their relative thermal stabilities [1], (b) we have quantified the effect of water adsorption on surface structure by tracking the surface phonon spectral response as a function of water chemical potential providing new experimental observables for the characterization of partially reconstructed oxide surfaces [2] and (c) we have shown that the α-Al2O3(0001)/liquid water interface is, at circumneutral pH, hydrophobic: that the first layer of adsorbed water does not donate hydrogen bonds to bulk liquid.
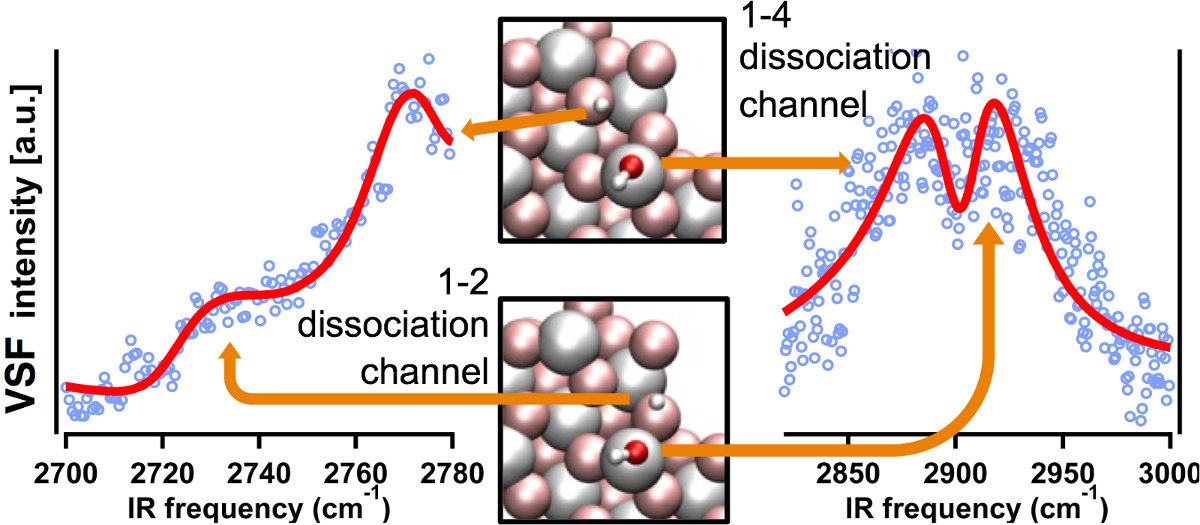
Figure 1: Summary showing the measured spectra of the two theoretically predicted unimolecular water dissociation pathways on the α-Al2O3(0001) surface in UHV.
[1] H. Kirsch et al., J. Phys. Chem. C 118(25), 13623-13630 (2014)
[2] Y. Tong et al., J. Chem. Phys. 142(5), 054704 (2015)