Poster
Molecular dehydrogenation reactions on surfaces
1Dpto. Física Aplicada I, Universidad del País Vasco UPV/EHU, San Sebastián, Spain
2Donostia International Physics Center (DIPC), San Sebastián, Spain
3Centro de Física de Materiales, CSIC-UPV/EHU, San Sebastián, Spain
4IKERBASQUE, Basque Foundation for Science, Bilbao, Spain
It is a well-know experimental issue that residual H is abundant in a seemingly ideal UHV environment [1], which can have a significant effect on STM and XPS measurements. For example, additional XPS peaks are often observed when evaporating organic molecules on a surface [2]. Although such features are commonly attributed to the splitting of the molecular levels originated by the symmetry-breaking of the system upon deposition on the substrate [3], they might also be induced by the presence of residual H and the consequent hydrogenation of the evaporated molecules.
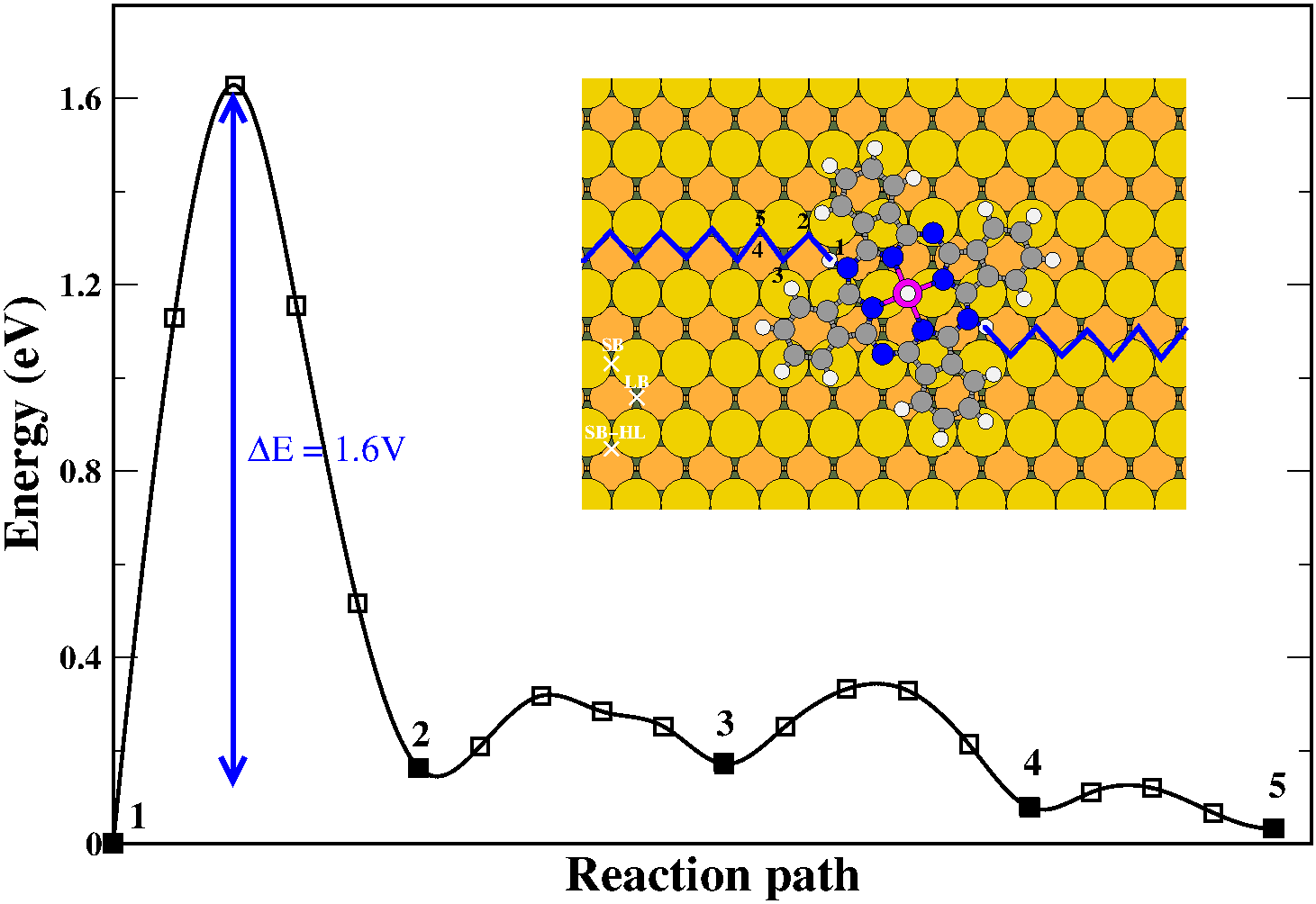
In this work, we combine density functional theory (DFT) and X-ray photoelectron spectroscopy (XPS) to study the adsorption of ZnPc molecules on Cu(110). Firstly, our calculations demonstrate that the double peak observed in the XPS experiments is not a final-state effect, i.e. is not due to splitting of the LUMO level. Rather, the reaction of the ZnPc molecules with extra H atoms in the chamber gives rise to H3ZnPc molecules, which having two inequivalent N atoms produce a double peak in the XPS spectra. Subsequently, we investigate the thermal evolution of the system and we conclude that the evolution of the XPS peaks upon annealing is related to the dehydrogenation of the H3ZnPc molecules following a two-step process.

Figure: Energetics along the diffusion path of H atoms on Cu(110) in the vicinity of a HZnPc molecule.
[1] F. D. Natterer et al., Surf. Sci. 615, 80 (2013)
[2] A. Garcia-Lekue et al., J. Phys. Chem C 116, 15378 (2012)
[3] P. Borghetti et al., ACS Nano 8, 12786 (2014)