Poster
Adsorption site dependence of vibrational excitations of molecular hydrogen
1CIC nanoGUNE, 20018 Donostia-San Sebastián, Basque Country, Spain
2IKERBASQUE, Basque Foundation for Science, 48011 Bilbao, Basque Country, Spain
3Centro de Física de Materiales, 20018 Donostia-San Sebastián, Basque Country, Spain
4Donostia International Physics Center, 20018 Donostia-San Sebastián, Basque Country, Spain
Transition-metal phtalocyanines are a well-known class of molecules used as model to study the interaction between metal surfaces and metal-organic compounds [1][2]. These kind of metal-organic complexes present a wide range of properties and functionalities which depend on the coordination of their central metal ion, such as magnetism or the adsorption of small gas molecules [3].
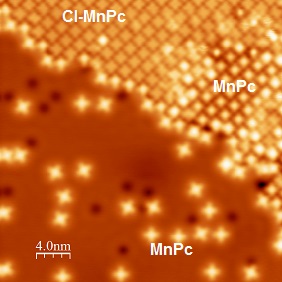
In this work we study Chlorinated Manganese Phtalocyanine (Cl-MnPc) molecules deposited on a Ag (111) substrate. We explore the adsorption characteristics of this system by means of a combined Low Temperature Scanning Tunneling and Atomic Force Microscope. After deposition on a room temperature substrate, a fraction of (dechlorinated) MnPc molecules coexist with Cl-MnPc on the surface. Moreover, Cl-MnPc can be controllably dechlorinated after the evaporation process. We find that both molecules are a preferential site of adsorption for molecular Hydrogen, which is known to present a bistable vibrationally mediated behavior depending on its different adsorption configurations [4]. Inelastic tunneling of electrons from a STM can excite such bistability which induces a fingerprint close to zero-bias on differential conductance measurements. Additionally, force spectra reveal differences on the electrostatic forces exerted between the tip and the molecule when the tunneling electrons trigger such hydrogen fluctuations. We find that these fingerprints are strongly modified by the presence or absence of Chlorine atoms in the phtalocyanine molecules.

Figure 1: STM image of a self assembled island of Cl-MnPc. MnPcs coexist both in the island and in the Ag (111) surface.
[1] A. Mugarza et al., Phys. Rev B 85, 155437 (2012)
[2] Ying-Shuang Fu et al., Phys. Rev. Lett. 99, 256601 (2007)
[3] K. Seufert et al., J. Am. Chem. Soc. 132, 18141-18146 (2010)
[4] C. Lotze et al., Science 338, 779 (2012)