Poster
Diffusion of H2O on a NaCl(100)
1Donostia International Physics Center (DIPC), P. Manuel de Lardizabal 4, Donostia 20018, Spain
2IKERBAQUE, Basque Foundation for Science, E-48011 Bilbao, Spain
3Leibniz Universität Hannover, Institut für Festkörperphysik, Abteilung für Atomare und Molekulare Strukturen (ATMOS), Appelstr. 2, D-30167 Hannover, Germany
4Ruhr-Universität Bochum, Institut für Physikalische Chemie I, Universitätsstr. 150, D-44801 Bochum, Germany
5Centro de Física de Materiales (CFM-MPC) CSIC-UPV/EHU, Paseo Manuel de Larizabal 5, San Sebastián 20018, Spain
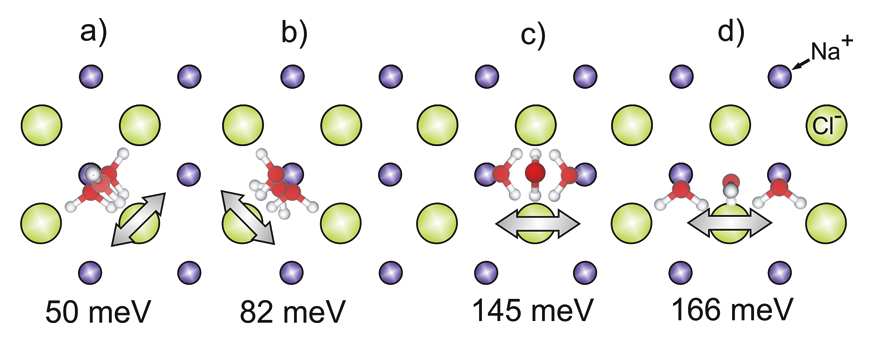
The motion of D2O monomers is investigated on a NaCl(100) bilayer on Ag(111) between 42.3 and 52.3 K by scanning tunneling microscopy and density functional theory [1]. In previous work, we did investigate the diffusion mechanism of H2O on NaCl(100) at low coverage. For that we determined the energy barriers associated with different hopping mechanisms, classified as translations and reorientations of the water molecule. The combination of these hopping mechanisms leads to net movement of the molecule along the surface with relatively low energetic cost, compared with bare parallel translation of the water molecule [2]. In the recent work the mechanism of the motion is identified by comparison of the experimental results to theoretical calculations. Via low temperature adsorption site determination in connection with density functional theory, we reveal an influence of the metallic support onto the intermediate state of the diffusive motion.

Figure 1: Top view of diffusion processes, (a) OH-flip, (b) parallel rotation, (c) O-flip, (d) higher O-flip. Small blue circles correspond to Na+, large green circles correspond to Cl-, red circles correspond to O atoms, and white circles correspond to H atoms.
[1] S. C. Heidorn, C. Bertram, P. Cabrera-Sanfelix, and K. Morgenstern, ACS Nano DOI: 10.1021 (2015)
[2] P. Cabrera-Sanfelix, A. Arnau, G. R. Darling, and D. Sanchez-Portal, J. Phys. Chem. B 110, 24559 (2006)